
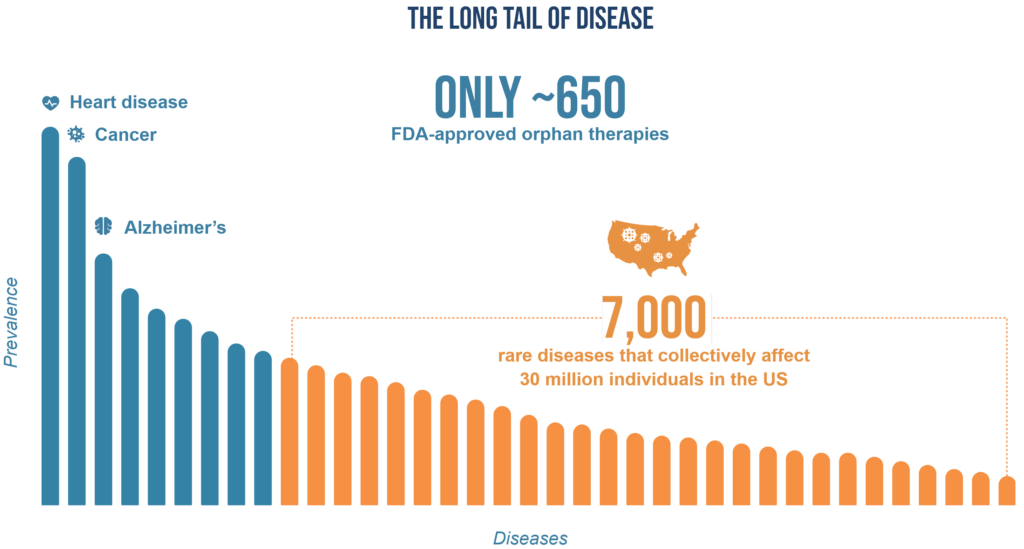
All of us know someone who has been affected by a prevalent condition like cancer or heart disease or Alzheimer’s disease. Most of us also know someone affected by one of many rare diseases, given that they collectively affect about 30 million people in the U.S. and 300 million people worldwide. There are approximately 7,000 rare diseases identified so far, yet there are only about 650 FDA-approved orphan drugs for these diseases. This represents an overwhelming treatment gap that leaves thousands of rare diseases and millions of patients in urgent need. These thousands of rare diseases are what we call “The Long Tail of Disease” and represent the great unmet need in our industry.
By Adam Rosenthal, PhD
CEO and Founder, Star Therapeutics
All of us know someone who has been affected by a prevalent condition like cancer or heart disease or Alzheimer’s disease. Most of us also know someone affected by one of many rare diseases, given that they collectively affect about 30 million people in the U.S. and 300 million people worldwide. There are approximately 7,000 rare diseases identified so far, yet there are only about 650 FDA-approved orphan drugs for these diseases. This represents an overwhelming treatment gap that leaves thousands of rare diseases and millions of patients in urgent need. These thousands of rare diseases are what we call “The Long Tail of Disease” and represent the great unmet need in our industry.
Star Therapeutics was founded on this mission: to develop life-changing therapies for as many rare diseases as possible for the millions of patients in urgent need. To fulfill this mission, we have looked at the challenge in a different way and taken a unique approach. While many drug development efforts start with a technology platform or a specific biological target or asset, we start with the disease, and not just one but multiple rare diseases that share a common pathobiology. This enables us to discover and develop therapeutics that can treat numerous diseases with a single therapy, representing pipeline-in-a-product opportunities and having a multiplier effect on the number of rare diseases we can treat.
One Disease on the Long Tail
I founded Star Therapeutics in 2018, motivated by my prior biotech experiences in rare diseases. At my last company, True North Therapeutics, we focused solely on rare diseases in hematology and immunology driven by the biology of the Complement system. Our lead indication was a rare hematologic condition called cold agglutinin disease (CAD).
Over five years ago when True North first started to work on CAD, it was a condition that lacked awareness and understanding. At the time, very little was known in the medical literature about CAD, except through a small number of published case studies, which gave the general impression of it being a mild anemia that often did not require any interventional therapies. However, when speaking to treating physicians and directly to patients, we heard stories that revealed a disease with significant morbidity and the potential to be life-threatening.
To address this disconnect, our team at True North set out to fill the information gap. We conducted several natural history studies in CAD and uncovered data that brought to light the severe nature of the disease and its true burden on patients’ lives. We advanced our lead program at True North from an idea through clinical proof-of-concept, after which our company and Complement programs were acquired by Bioverativ in 2017 and subsequently by Sanofi in 2018. Through the heroic efforts of these three companies, ENJAYMO (sutimlimab) recently received FDA approval on February 4, 2022 as the first approved treatment for CAD.
Looking at the Horizon Ahead
Similarly to CAD, most of the approximately 7,000 rare diseases go unnoticed without adequate treatment and carry a much higher burden to patients than is generally recognized. It is up to companies like us to do the work and figure it out – to understand the patient journey, to uncover the true burden of disease, and to establish the benefit of treatment.
At Star Therapeutics, we view the thousands of rare diseases like stars in the night sky, each one representing an unknown entity that we hope to fully understand one day. Individually, the vast number of stars in the sky can seem overwhelming, but the early astronomers saw things differently and that certain stars formed constellations like The Big Dipper or Orion. We take a similar approach at Star when viewing the thousands of rare diseases, finding diseases that share a common pathobiology that can be grouped together and potentially treated in the same way.
My first hire at Star was Sandip Panicker, our Chief Scientific Officer, who I had the pleasure of working with at two prior companies. Our initial program ideas were each homegrown with a first-in-class therapeutic approach, and each going after a constellation of diseases. We started operations in early 2019, building out a team of world-class drug hunters and drug developers that makes Star truly special. Over the years, we have built an environment of excellence, diversity, creativity, and passion that supports our team and mission to develop life-changing therapies for patients with rare diseases.
I am excited by the next phase of Star’s journey, as we continue to build our team and expand our pipeline into other diseases and new programs. Our initial programs started from scratch, with novel biology interrogation and antibody discovery, and have since progressed rapidly. Our first program, which is part of Electra Therapeutics, is now in the clinic, less than three years from initiation. In the end, the only real measure of success in our industry is whether we can cross the finish line and get a novel therapy into the hands of patients. I am hopeful that, with our unique therapeutic insights and our seasoned team, Star will get there many times over.

