© 2025 STAR Therapeutics Inc.

Providing Solutions for Patients With Bleeding Disorders, Starting With VWD
Our VEGA Program starts with VGA039, a subcutaneously administered therapy for patients living with bleeding disorders such as VWD.
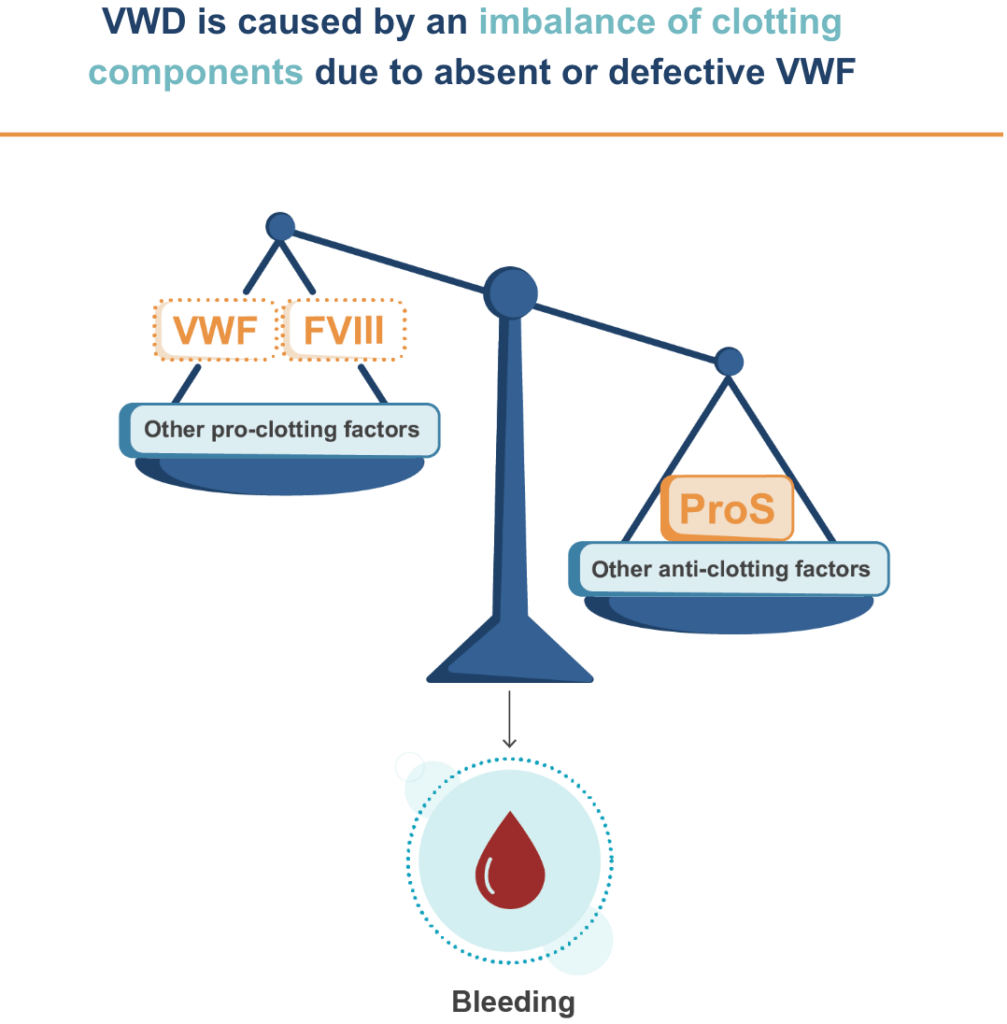
VWD is often overlooked and underdiagnosed. Despite being the most common bleeding disorder, VWD receives less focus and research than other bleeding disorders, such as hemophilia A. As a result of lower awareness, increased time to diagnosis, and a lack of treatment innovation, many people with VWD remain undiagnosed or not properly treated.1,2
Current VWD treatments have significant limitations, including few prophylaxis options and high treatment burden.1,3 Some patients miss school or work due to bleeding or treatments that require frequent IV infusions.4 Many patients have severe bleeding that leads to anemia and blood transfusions. VWD can mean heavy bleeding during menstruation and childbirth, and a 10x higher risk of mortality.1,5,6

We believe that VWD patients deserve a better and more convenient treatment option, such as a subcutaneous therapy, which can prevent and manage bleeding and improve quality of life.

We believe VWD patients deserve better and more convenient treatment options, such as subcutaneous therapy, which can prevent and manage bleeding and improve quality of life.
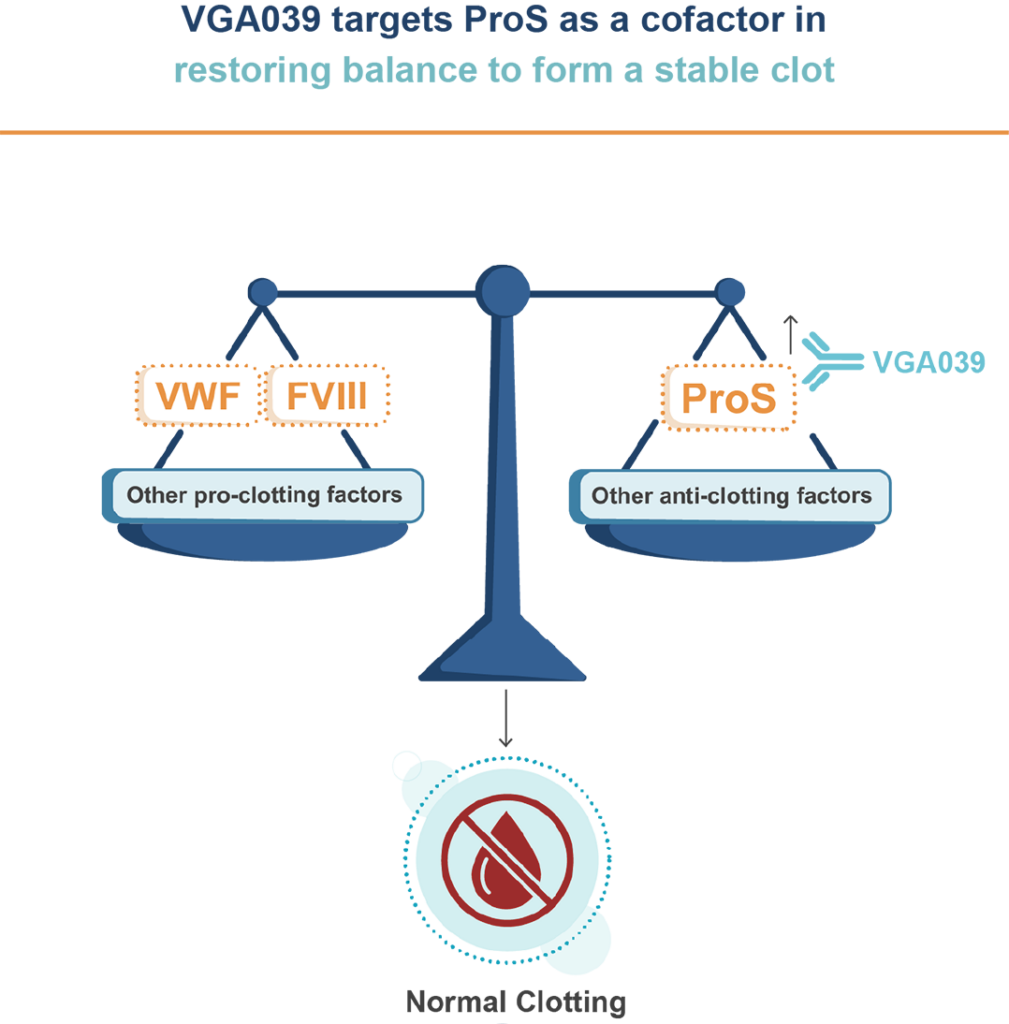
Our first-in-class Protein S—targeting antibody, VGA039,
has the potential to improve the lives of people living with VWD.
VGA039 is a monoclonal antibody that targets Protein S (ProS), a key component in restoring balance to the blood clotting process. This helps balance the coagulation components needed to promote blood clotting in people living with VWD, offering the potential to transform their treatment experience.

For more information about the ongoing trial and to sign up for updates
For more information about enrolling in current clinical trials, reach out to us.
Star does not currently accept or grant requests for expanded access to any of the VEGA Program’s investigational therapies outside of clinical trials.
References: 1. Centers for Disease Control and Prevention. What is Von Willebrand Disease? April 1, 2021. Accessed September 26, 2022. https://www.cdc.gov/ncbddd/vwd/facts.html 2. Sidonio RF Jr, Zia A, Fallaize D. Potential undiagnosed VWD or other mucocutaneous bleeding disorder cases estimated from private medical insurance claims. J Blood Med. 2020;11:1-11. doi:10.2147/JBM.S224683 3. Denis CV, Susen S, Lenting PJ. von Willebrand disease: what does the future hold? Blood. 2021;137(17):2299-2306. doi:10.1182/blood.2020008501 4. Holm E, Carlsson KS, Lövdahl S, Lail AE, Abshire TC, Berntorp E. Bleeding-related hospitalization in patients with von Willebrand disease and the impact of prophylaxis: results from national registers in Sweden compared with normal controls and participants in the von Willebrand Disease Prophylaxis Network. Haemophilia. 2018;24(4):628-633. doi:10.1111/hae.13473 5. Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6(1):228-237. doi:10.1182/bloodadvances.2021005589 6. Castaman G, James PD. Pregnancy and delivery in women with von Willebrand disease. Eur J Haematol. 2019;103(2):73-79. doi:10.1111/ ejh.13250
© 2025 STAR Therapeutics Inc.
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |

We look forward to connecting with you